Request a free sample copy@ https://www.persistencemarketresearch.com/samples/10628
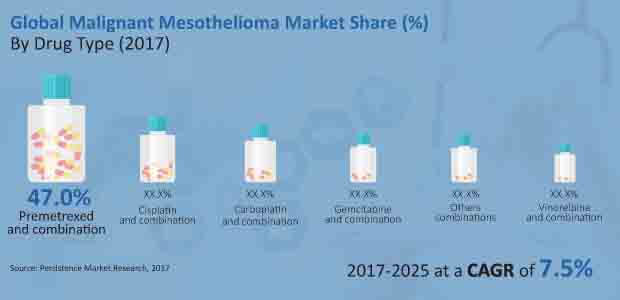
Due to the superior response rate of pemetrexed, which ranges between 30% and 40%, whether used alone or in combination with other drugs, the market share of pemetrexed and combination products is expected to increase by more than 300 BPS by 2025 compared to 2017 climb.
In terms of revenue, pemetrexed and the combination drug segment dominated the global malignant mesothelioma market in 2016 and this trend is expected to continue throughout the forecast period. The pemetrexed and combinations segment is also the most attractive with a market attractiveness score of 3.2 over the expected lifetime.
Contact sales for additional purchase assistance [email protected]
https://www.persistencemarketresearch.com/checkout/10628
Market Taxonomy : Malignant Mesothelioma Market
-
- Malignant Mesothelioma Market Type of drug: pemetrexed, cisplatin, carboplatin, Gemcitabine, vinorelbine, Other
- Malignant Mesothelioma Market route of administration: Orally, parenterally
- Malignant Mesothelioma Market Region : North America, Latin America, Europe, APAC, THING
- Malignant Mesothelioma Market distribution channel : Hospital pharmacies, retail pharmacies, Oncology Centers
Customization request @ https://www.persistencemarketresearch.com/request-customization/10628
Companies covered in this report – Malignant Mesothelioma Market
-
- AstraZeneca Plc.
- Bristol-Myers Squibb Company
- Hoffmann-La Roche Ltd.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Eli Lilly and Co
- Teva Pharmaceuticals
- Boehringer Ingelheim GmbH
- Mylan NV
- Fresenius Kabi AG
- Sun Pharmaceuticals Industries Ltd
- Corden Pharma International GmbH
- Concordia International Corp
- Kyowa Hakko Kirin Co.Ltd.
- Polar Pharmaceuticals, Inc.
- MolMed SpA
- Ono Pharmaceutical Co.Ltd
- Nichi-Iko Pharmaceutical Co.,Ltd
Asbestos exposure is believed to lead to malignant mesothelioma and…
source_link https://www.digitaljournal.com/pr/malignant-mesothelioma-market-grow-on-a-decisive-note-by-2023