[ad_1]
Today, the Office of Generic Drugs (OGD) at the Food and Drug Administration (FDA) released its 2021 Annual Report. As noted in the report, 90% of all drugs dispensed in the US are generic. In 2021, there were 776 approved generic drugs through the Abbreviated New Drug Applications (ANDA) process. Approvals by month are below.
Out of these 776, 93 were first generic drugs. A list of first generic approvals that the FDA OGD defined as “significant” is listed below.
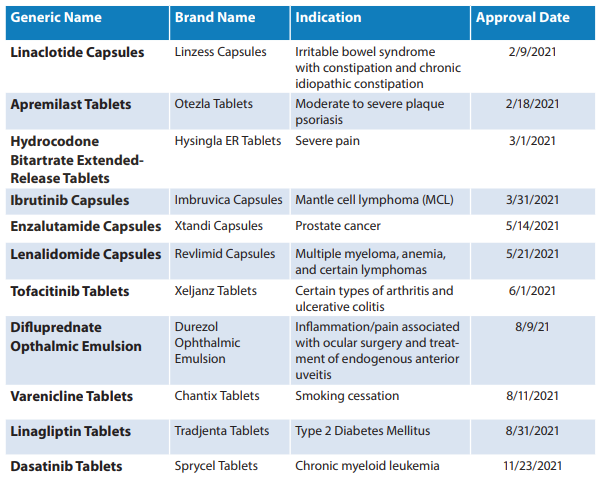
The OGD report is an important reminder “…that the purpose of the pharmaceutical industry is to create a ‘mountain of inexpensive generics‘ to help humanity in perpetuity “
[ad_2]
Source link








